An atom is the smallest unit of matter that forms a chemical element. Everything in nature, from the air we breathe to the cells in your body, are made of atoms. That means every state of matter, whether solid, liquid, gas, or plasma, contain atoms. They are usually neutral, meaning they do not have a positive or negative charge, and they are made up of smaller particles called protons, neutrons, and electrons. All of these particles are extremely small and invisible to the naked eye.
The concept of a tiny particle that makes up the matter we see around us dates back to the Ancient Greeks. It’s from them that we get the word atom, which means “uncuttable” in the language. Their understanding of atoms was a philosophical concept, though, and we have learned much since then due to centuries of scientific analysis. In the 1800s, John Dalton was the first person to adapt theories about atoms into the first modern atomic model. In his model, he devised that all matter consists of tiny particles called atoms, that atoms are indestructible and unchangeable, that the same elements will have the same characteristics such as mass or size, and that when elements react, their atoms combine to form new compounds.
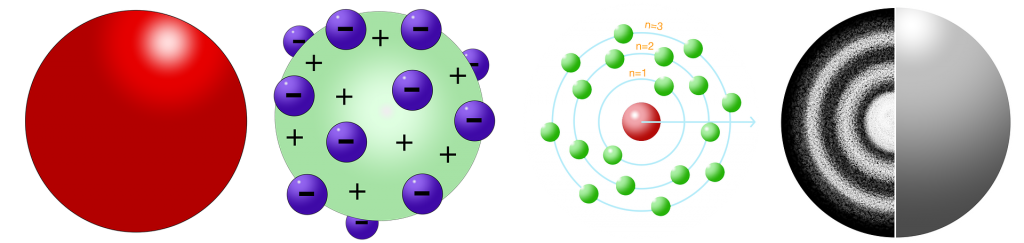
In 1890s, J.J. Thompson discovered the negatively charged particles that would later become known as electrons were a part of atoms. He came up with the plum pudding atomic model where each atom is a sphere filled with a positively-charged fluid filled with the negatively charged electrons.
Source: Wikimedia Commons
In the 1910s, Ernest Rutherford conducted an experiment to further determine the structure of the atom. By firing positively – and negatively-charged particles at gold foil and observing how many of the particles deflected, he was able to determine that atoms had a dense, positively-charged center called a nucleus. He created a new model of the atom, called the Planetary Model, where a positively-charged nucleus existed at the center of each atom and electrons orbited the nucleus. To explain why the electrons weren’t attracted to the positively-charged nucleus, he stated that electrons orbited the nucleus in a wide orbit just like the planets in our solar system orbit the Sun. A few years later, Niels Bohr would clarify Rutherford’s model, stating that electrons orbit the nucleus in orbits that have a set size and energy. Though electrons do not follow any particular orbit at all times, lower energy electrons will fill the lowest energy orbitals. Once one level is filled, a new level begins.
The current model of the atom is called Atomic Cloud or Electron Cloud Model. Erwin Schrödinger came up with this model in the 1920s. It kept the same framework as the Planetary Model but modified it a bit. In Schrödinger’s model, the atom still consists of a dense nucleus surrounded by electrons, but the electrons do not travel in an exact orbit. Using Heisenberg’s Uncertainty Principle, he realized it was impossible to know exactly where the location of an electron would be; we could only predict where it should be. This model is called the Electron Cloud Model because the electrons are shown to exist in regions around the nucleus that resembles a cloud.
Summary
- An atom is the smallest unit of matter that makes up a chemical element.
- All matter is made up of atoms.
- The word atom comes from Greek, meaning “uncuttable.”
- John Dalton came up with the first modern model of an atom.
- J.J. Thompson discovered the first subatomic particle – the electron – in the 1890s. He created the Plum Pudding Atomic Model.
- In the 1910s, Ernest Rutherford discovered the nucleus within atoms and realized it was positively charged. He created the Planetary Atomic Model.
- Along with Rutherford, Niels Bohr clarified the Planetary Model, stating that electrons did not remain in set orbits.
- In the 1920s, Erwin Schrödinger, realizing it was impossible to determine the location of electrons, came up with the Electron Cloud Atomic Model where the approximate region of the electron may be known but not the exact position. This is the current model used today.
Looking for ways to practice your Chemistry knowledge? Check out our Chemistry worksheets! If you need more help, you can also contact us to get one on one, private tutoring.
Looking for extra practice? Try our resources:
Related Resources: How To Read The Periodic Table Explainer
View our resources
Strive Resources | TpT | Made By Teachers | Classful | Etsy