Table of Contents
- What is an element?
- Atomic number
- Chemical symbol and name
- Atomic mass
- Charge
- Groups
- Periods
- Conclusion
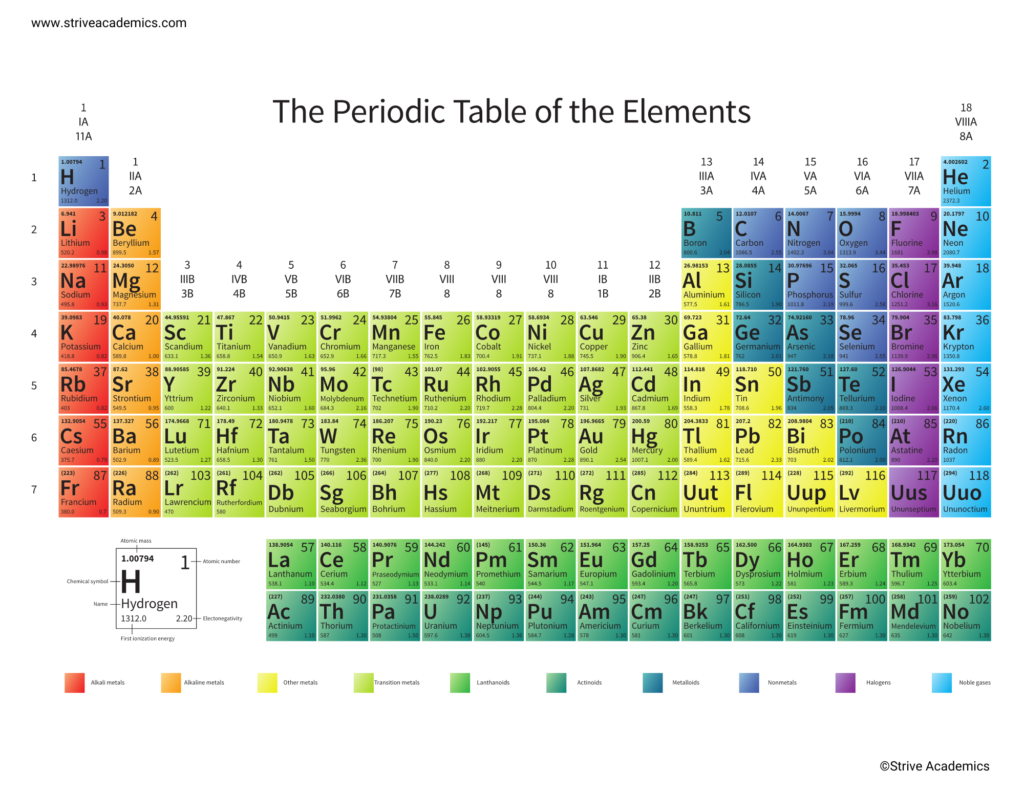
Reading the Periodic Table can seem to be very confusing at first. It’s filled with a bunch of numbers and symbols. At first, it seems like there’s no telling what it could all mean. When you learn more about how the Periodic Table is organized, though, you will see that it shows you a lot of useful information about elements.
Look at the diagram above. It presents an enlarged version of one of the boxes you will see on the Periodic Table. The information in this box is a little simplified. You will see that some periodic tables will give you much more information, but, for now, we will stick to the basics. This diagram shows you the key details you will need to identify when looking at an element on the Periodic Table: its atomic number, chemical symbol, chemical name, atomic mass, and some other details you won’t need to know immediately. Note that the periodic table above has a slightly different key, and the atomic mass is on top, next to the atomic number. These things help you tell the different elements apart from each other. On the next page, we will discuss in detail what each of these things mean.
You will also notice that the elements on the periodic table are color-coded. Elements are organized into groups that share similar characteristics. If you see elements that have the same color, it means that they act in similar ways under the same conditions. For example, if an element is in the Alkali Metals group, we expect them to be extremely reactive and combine with other elements easily, but if it’s in the Noble Gas group, they will not react with other elements easily.
What is an Element?
An element is a pure substance that is made up of only one kind of atom. That means all of the elements that are alike will have atoms with the same structure and characteristics. Atoms of the same element will have the same number of protons in their nucleus. Usually, the number of neutrons in an element’s atom will be the same, but sometimes there are differences. Atoms of the same element that have a different amount of neutrons than what is most common in nature are called isotopes. You won’t see these variations listed on the Periodic Table, but the atomic mass that you see for various elements hints at these isotopes because it is an average of the most common masses for that element in nature.
An element of any substance cannot be broken down into simpler substances by ordinary chemical processes. Roughly 90 of these elements occur naturally on Earth. Using technology, however, scientists have been able to create additional elements that do not naturally occur. Modern Periodic Tables recognize 118 elements.
Atoms of alike or different elements will combine to form molecules, a group of atoms, in chemical reactions. When elements combine, they create new substances such as water or steel. Sometimes various elements and substances get mixed together to make up things like air or rocks.
Atomic Number
The atomic number of an element represents the number of protons within the atom of that element. A proton is a positively charged particle in the center of the atom, its nucleus. Protons only make up one part of the atom’s nucleus. You will also find neutrons, particles with no charge, in the center of the atom as well. The atomic number will not help you in identifying the number of neutrons in an element, though.
Stable elements will have a neutral charge, so the positively charged particles and negatively charged particles should be in balance. Because of this phenomenon, we can also use the atomic number to identify these negatively charged particles, electrons, within an atom. If the element has a charge of 0, the number of its electrons will be identical to the atomic number.
Another thing you should know is that an element’s atomic number will never change. Other things, like the number or neutrons and protons make differ in many different ways within one element, but its number of protons will never change. If you change the amount of protons, you will end up with a completely different element!
Chemical Symbol and Name
Just as important as the atomic number of an element is the name we call it and the symbols we use to refer to it. Every element on the Periodic Table has its name listed along with a shorter symbol that we use to refer to it when writing out chemical formulas. It’s important to learn the names and symbols of these elements – along with their approximate position on the table – so that you can find them easily when you need to talk about them.
Some of the most common elements you will talk about include hydrogen (H), helium (He), sodium (Na), iron (Fe), carbon (C), nitrogen (N), oxygen (O), and chlorine (Cl). You come into contact with many of these elements on a daily basis, so they’re a great way of introducing you to elements and chemical reactions.
You might also notice that some of the symbols for these elements do not exactly line up with their given name. That’s because the symbols for some of the elements are based off of their Latin names because they were known in ancient times. Iron, for example, as the symbol Fe because its Latin name was ferrum.
Atomic Mass
Atomic mass is represented by the number at the bottom of each square for an element on the Periodic Table. It measures the total mass of an atom of that element in atomic mass units or AMU. As mentioned above, the nucleus of an atom is made up of protons and neutrons. Each of these particles has an atomic mass unit of 1. If an atom has 5 protons and 5 neutrons, its total amu would be 10.
Now you might be wondering “What about electrons?” They’re included in atoms too, but electrons are so tiny compared to a proton or neutron that their mass does not show up easily when calculating in amus. Because of this, atomic mass is not very useful for identifying the number of electrons within an atom. Instead, refer to the atomic number.
Atomic mass is very helpful for identifying the number of neutrons within an atom, though. Since the atomic mass is calculated by looking at the protons and neutrons within an atom, you can calculate the number of neutrons within an atom by subtracting the atomic number (protons) from the atomic mass (protons and neutrons). We mentioned before that all atoms of the same element must have the same number of protons. This is not the case for neutrons, though! The number of protons and neutrons are not always equal within an atom, so it sometimes becomes necessary to identify these differences when they occur.
If you look carefully at some periodic tables, you will notice that the atomic mass is listed as a decimal. Why is that when you can’t have part of a proton or neutron? It is because those numbers are based on the average of the most common type of that element found in nature. All elements come in variations with slightly different numbers of neutrons. These variations are called isotopes. These isotopes act the same chemically but may have different physical properties. Some of these isotopes may even be radioactive.
Charge
Most atoms are normally neutral in charge, but when they gain or lose electrons, they will develop a negative or positive charge. The Periodic Table helps you determine the charge an element would normally have when involved in chemical reactions.
Starting with Group I, elements have a positive charge. Look at the last digit in the number to determine the charge. Group 1 has a +1 charge. Group 2, +2. Skip the Transition Metals (Groups 3 – 12) because elements in that region may have a variety of charges. Group 13 has a +3 charge, and Group 14 may have a + or – 4 charge. Starting with Group 15, the charges are negative, starting with a -3 charge and decreasing to 0 by Group 18.
This information becomes important when you want to determine how elements combine with one another for form ionic bonds. These bonds occur when charged atoms or ions are attracted to one another and combine. One easy method to determine how two ions will combine is to assign the charge to the opposite element. The charge becomes the number of that element needed in the combination to make the resulting compound neutral. In the example below, Be has a charge of +2 and Cl has a charge of -1. That means that to combine, you need 2 Cl atoms to balance the +2 charge of Be. The resulting compound is BeCl2.
Groups
A group on the periodic table refers to the atoms lined up vertically in columns on The Periodic Table. Elements within the same group have similar physical or chemical characteristics because their outer electron shells are similar. We’ve already talked about one of these characteristics – the charges typically observed from an element in a particular group – but other similarities exist as well. For example, elements in Group 2, which is pictured to the left, have a gray-white luster when freshly cut but quickly tarnish when exposed to air. They are good conductors of electricity and have higher melting and boiling points than the alkali metals in Group 1. Group 17 elements, on the other hand, are extremely reactive to the point where they are not found by themselves in nature. They’re also fairly toxic, form acids when combined with Hydrogen, and exist as diatomic molecules (two atoms) when in their pure form. Groups of atoms also have names, like mentioned above, and are color-coded on The Periodic Table to show that they have similar characteristics.
Periods
A period refers to a row of chemical elements on The Periodic Table. Elements in the same row have the same number of electron shells. Each element in the row has one more proton in its atoms and is less metallic than the element to the left of it.
Conclusion
The Periodic Table is a remarkable tool that can give you a wealth of information about elements and their chemical properties at a glance. Some periodic tables may be more advanced than others, but even the most simple periodic tables tell you a lot about an element and its properties once you learn how to read it. As you learn more about chemistry and the reactions or properties of the elements, The Periodic Table will be invaluable to your understanding of these concepts.
View our resources
Strive Resources | TpT | Made By Teachers | Classful | Etsy